Abstract
Chronic myeloid leukemia (CML) is a hematopoietic stem cell malignancy driven by BCR-ABL1 tyrosine kinase and effectively managed with tyrosine kinase inhibitors (TKIs) such as imatinib. Reactivation of BCR-ABL1 through mutations in the kinase is a common mechanism of resistance, but fails to explain clinical resistance in a significant proportion of cases, a situation referred to as BCR-ABL1-independent resistance. Additionally, most patients require continuous TKI therapy to avoid recurrence of active leukemia, suggesting that primitive, fully leukemogenic CML stem cells (LSCs) are BCR-ABL1-independent. We have previously demonstrated that BCR-ABL1-independent CML cells from patients with overt resistance require functional nucleocytoplasmic export (NCE) and are sensitive to knockdown of the NCE regulator RAN and selective inhibitors of NCE (SINEs) such as the CRM-1 inhibitor KPT-330 (selinexor, Karyopharm) [Khorashad et al. Blood. 2015;125(11):1772-81]. We hypothesize that reliance on NCE may extend to LSCs, the reservoir for persistent CML. To test this, we performed in vivo and in vitro experiments to evaluate the effects of a prototype SINE, KPT-330, on LSCs (defined as CD34+CD38-) and leukemic progenitor cells (LPCs, CD34+CD38+) isolated from newly diagnosed chronic phase CML patients and normal cord blood (CB).
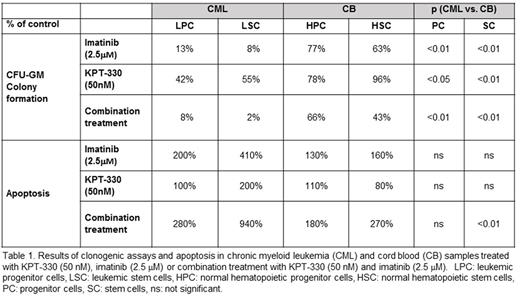
Treatment with KPT-330 (50 nM) reduced colony formation by CML (n=4) LPCs and LSCs to 42% and 55% of controls, compared to 78% and 96% for CB (n=3) (Table 1). When combined with imatinib (2.5 µM), KPT-330 treatment dramatically reduced colony formation by LPCs and LSCs to 8% and 2% of controls, compared to 66% and 43% for CB. Single agent KPT-330 (50 nM) had modest effects on apoptosis in LSCs and LPCs, but the combination increased apoptosis in LPCs and LSCs by 280% and 940% relative to controls, compared to 180% and 270% respectively for CB (Table 1). These data suggest that combining KPT-330 with imatinib has the most profound effects on LSCs, with a large differential compared to normal hematopoietic stem cells.
To test the effect of KPT-330 on functionally defined LSCs, CML CD34+ cells were cultured ex vivo with or without KPT-330 (50 nM) and/or imatinib (2.5 µM) for 72 hours, followed by plating in long term culture-initiating cell (LTC-IC) assays (n=5) or injection into NSG mice (n=20, 5 per treatment arm). While no reduction in colonies was seen in LTC-IC experiments with single agent KPT-330 or imatinib, a 64% reduction in LTC-IC colonies was observed with combination treatment (p<0.05). Genotyping of LTC-IC colonies by fluorescent-in-situ-hybridization (FISH) revealed that BCR-ABL1+ colonies were reduced by 11% and 22% relative to controls, when treated with KPT-330 or imatinib respectively, but combination treatment reduced BCR-ABL1+ colonies by 38%, significantly lower than either single agent (p<0.05). Thus far results are available from one xenograft experiment. All treatment groups engrafted with a median of 80% human CD45+ cells in bone marrow and 54% in spleen at 12 weeks after injection. Human CD45+ cells were sorted and genotyped. The median proportion BCR-ABL1+ cells in the control was 40%, and was not significantly reduced by imatinib or KPT-330 alone. However combination treatment reduced BCR-ABL+ cells by 42% (p<0.01).
To identify potential CRM1 clients regulated in LSCs, we performed a meta-analysis of published gene expression data on CML vs. normal C34+38- or quiescent cells. Candidate genes with differential expression between CML and normal were cross-referenced against putative CRM1 cargos predicted by the LocNES tool [Xu et al. Bioinformatics. 2015;31(9):1357-65]. Confocal microscopy is ongoing to validate cytoplasmic relocalization upon KPT-330 exposure for several candidates, including CD26, an established marker of LSCs that has recently been shown to enhance quiescence in hematopoietic cells [O'Leary et al. Leukemia. 2017 Apr 21;Epub ahead of print].
Our data illustrates that interrupting nucleocytoplasmic export by SINEs preferentially eliminates CML LSCs and enhances their sensitivity to TKIs, suggesting that combinations of TKI and SINEs represent a clinical strategy to target BCR-ABL1 independent resistance, as well as persistent residual CML. Mechanistic studies exploring the effects of nuclear entrapment of certain NCE cargo proteins of biological significance in CML LSCs are ongoing and will be reported.
Deininger: Celgene: Research Funding; Incyte: Consultancy; Novartis: Consultancy, Research Funding; ARIAD: Consultancy; Gilead: Research Funding; Pfizer: Consultancy; BMS: Consultancy, Research Funding; Ariad Pharmaceuticals, Bristol Myers Squibb, CTI BioPharma Corp, Gilead, Incyte, Novartis, Pfizer, Celgene, Blue Print, Galena: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal